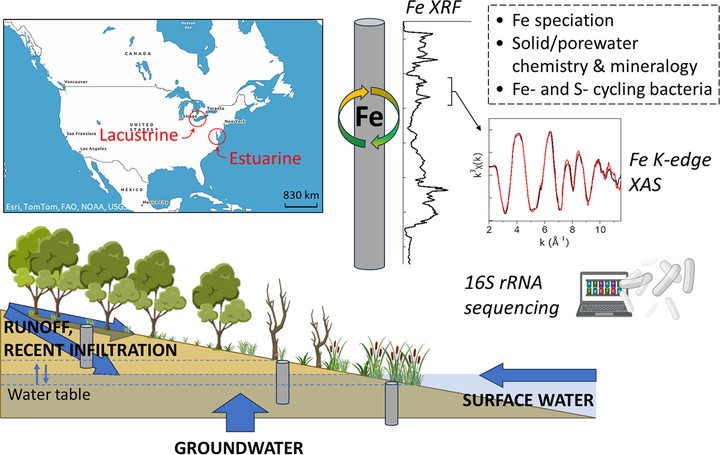
Biogeochemical controls on iron speciation and cycling across upland to shoreline gradients in freshwater and estuarine coastal soils (Lake Erie and Chesapeake Bay, United States)
Abstract
Coastal environments are dynamic interfaces that mediate carbon and nutrient exchanges between terrestrial landscapes and open waters, and understanding the biogeochemical factors controlling these exchanges, particularly iron (Fe) redox transformations, is crucial for predicting coastal ecosystem functions. Here, we investigated the mechanisms controlling Fe speciation changes across upland-to-shoreline gradients in freshwater and estuarine soils using Fe K-edge X-ray absorption spectroscopy, solid and porewater composition analysis, and 16S rRNA sequencing analysis. We show that Fe transformations depend primarily on inundation patterns. In unsaturated uplands, Fe occurs as Fe(III) oxyhydroxides, mainly goethite (9–35 %), Fe(II,III)-phyllosilicates (39–89 %), and Fe(III)-organic species (0–61 %). Soils influenced by estuarine waters exhibit porewater sulfide concentrations reaching up to 221 μM, Fe- and S-cycling bacteria, and up to 81 % pyrite (FeS2), indicating that sulfur-driven redox dynamics control Fe transformations. In lacustrine wetlands, Fe(III) reduction is indicated by porewater Fe(II) concentrations increasing to 1.0–2.1 mM, and ~10–15 % of Fe as Fe(II,III)-(hydr)oxides (green rust), vivianite (Fe3(PO4)2·8H2O), and/or adsorbed Fe(II) species. EXAFS data also indicate reduction of structural Fe(III) to Fe(II) in phyllosilicates. The presence of Fe- and S-cycling bacteria, as well as sulfide (0–10 μM), suggests that Fe-cycling is microbially driven and potentially coupled with cryptic S-cycling. Fe(II) oxidation was indicated above/near the water table by the presence of Fe(III) oxyhydroxides (ferrihydrite, lepidocrocite). Furthermore, negligible Fe(III) or sulfate reduction was observed at some water-saturated sites located at the upland-wetland transition, likely due to oxic (sub-)surface water inputs. Overall, our results highlight the importance of considering both Fe-speciation and hydro-biogeochemical dynamics when predicting Fe-cycling at coastal interfaces.